Centre for Environment Education and Technology (CEET)
Kiranam, Arpookara East P.O.
Kottayam, Kerala, India - 686008
P: +91 2594458, +91 9447 779 269 | E: ceet.india@gmail.com
Kiranam, Arpookara East P.O.
Kottayam, Kerala, India - 686008
P: +91 2594458, +91 9447 779 269 | E: ceet.india@gmail.com
Don’t forget to wash your hands!!!
The so called endocrine disrupting chemicals (Hormone mimicking) has variety of sources. They may easily enter into our body through both dermal and dietary exposures. Some of the chemical from this category may easily mark their entry into human body. Chemicals from bisphenol family are perhaps the best example. Bisphenols are plastcizers primarily used in the production of polycarbonate plastics and the resin lining of food and beverage cans. Bisphenol A (BPA), the most prominent one among them mark its presence in air, water, sewage sludge, soil, dust, foodstuffs, soft drinks and in human secretions. Regulations on its usage by the United States Environmental Protection Agency (USEPA) and the European Food Safety Authority (EFSA) made the entry of BPA analogues Bisophenol B(BPB), Bisphenol F(BPF), Bisphenol S (BPS) and Bisphenol AF (BPAF). Soon these alternatives also marked their presence in human body showing negative health impact. So for definite we have to avoid using polycarbonate plastic food containers (don’t heat them at least), and canned food stuffs. But one must also be careful in handling paper and paper products (including thermal receipts, flyers, magazines, tickets, mailing envelopes, newspapers, food contact papers, food cartons, airplane, boarding passes, luggage tags, printing papers, business cards, napkins, paper towels, and toilet paper) .
Now if such chemicals get into body and magnify, one may also expect physiological consequences. To know more read the following….. dx.doi.org/10.1021/jp500404u | J. Phys. Chem. B 2014, 118, 3832−3843 Exploring the Interaction of Bisphenol-S with Serum Albumins: A Better or Worse Alternative for Bisphenol A?
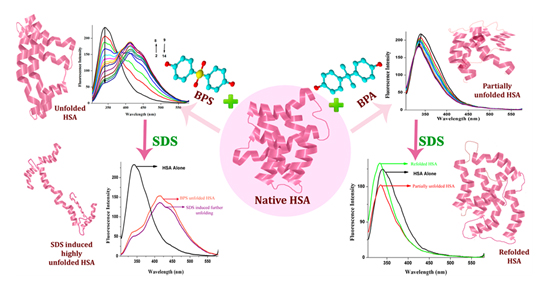
Now if such chemicals get into body and magnify, one may also expect physiological consequences. To know more read the following….. dx.doi.org/10.1021/jp500404u | J. Phys. Chem. B 2014, 118, 3832−3843 Exploring the Interaction of Bisphenol-S with Serum Albumins: A Better or Worse Alternative for Bisphenol A?

Abstract: The interaction of bisphenol-S (BPS) with serum albumins using steady-state, synchronous, time resolved, and circular dichroism spectroscopies has been investigated. The binding interactions have also been investigated in the case of bisphenol A (BPA). The fluorescence quenching pathways are different for both of these endocrine disrupting compounds. Steady-state and time resolved studies reveal static quenching at lower concentrations of BPS and dynamic quenching at higher concentrations. CD results also maintained the concentration dependent variation with a complete distortion of α-helices at
10−5M BPS. Besides this, addition of sodium dodecyl sulfate (SDS) results in the further unfolding of protein in the case of BPS, whereas time-resolved studies indicated refolding for BPA denatured human serum albumin (HSA). The entire study indicates an irreversible binding of BPS with HSA. Hence, these results reveal the possible involvement of BPS in the physiological pathway raising a health threat as already their presences in body fluids are known.
doi:10.1016/j.chemosphere.2011.06.060, J.E. Cooper et al. / Chemosphere 85 (2011) 943–947 Assessment of bisphenol A released from reusable plastic, aluminium and stainless steel water bottles
Abstract: Bisphenol A (BPA) is a ubiquitous high volume industrial chemical that is an estrogen and an environmental endocrine disrupting chemical. Bisphenol A is used extensively in the production of consumer goods, polycarbonate plastics, epoxy resins and coatings used to line metallic food and beverage cans. There is great concern regarding the possible harmful effects from exposures that result from BPA leaching into foods and beverages from packaging or storage containers. The objective of this study was to independently assess whether BPA contamination of water was occurring from different types of reusable drinking bottles marketed as alternatives to BPA-containing polycarbonate plastics. Using a sensitive and quantitative BPA-specific competitive enzyme-linked immunosorbent assay we evaluated whether BPA migrated into water stored in polycarbonate or copolyester plastic bottles, and different lined or unlined metallic reusable water bottles. At room temperature the concentration of BPA migrating from polycarbonate bottles ranged from 0.2 to 0.3 mg L-1. Under identical conditions BPA migration from aluminium bottles lined with epoxy-based resins was variable depending on manufacturer ranging from 0.08 to 1.9 mg L-1. Boiling water significantly increased migration of BPA from the epoxy lined bottles. No detectable BPA contamination was observed in water stored in bottles made from Tritan™ copolyester plastic, uncoated stainless steel, or aluminium lined with EcoCare™. The results from this study demonstrate that when used according to manufacturers’ recommendations reusable water bottles constructed from ‘‘BPA-free’’ alternative materials are suitable for consumption of beverages free of BPA contamination.
dx.doi.org/10.1021/es202507f | Environ. Sci. Technol. 2011, 45, 9372–9379 Widespread Occurrence of Bisphenol A in Paper and Paper Products: Implications for Human Exposure Abstract: Bisphenol A (BPA) is used in a variety of consumer products, including some paper products, particularly thermal receipt papers, for which it is used as a color developer. Nevertheless, little is known about the magnitude of BPA contamination or human exposure to BPA as a result of contact with paper and paper products. In this study, concentrations of BPA were determined in 15 types of paper products (n = 202), including thermal receipts, flyers, magazines, tickets, mailing envelopes, newspapers, food contact papers, food cartons, airplane boarding passes, luggage tags, printing papers, business cards, napkins, paper towels, and toilet paper, collected from several cities in the USA. Thermal receipt papers also were collected from Japan, Korea, and Vietnam. BPA was found in 94% of thermal receipt papers (n = 103) at concentrations ranging from below the limit of quantitation (LOQ, 1 ng/g) to 13.9 mg/g (geometric mean: 0.211 mg/g). The majority (81%) of other paper products (n = 99) contained BPA at concentrations ranging from below the LOQ to14.4 μg/g (geometric mean: 0.016 μg/g). Whereas thermal receipt papers contained the highest concentrations of BPA (milligram-per-gram), some paper products, including napkins and toilet paper, made from recycled papers contained microgram-per-gram concentrations of BPA. Contamination during the paper recycling process is a source of BPA in paper products. Daily intake (DI) of BPA through dermal absorption was estimated based on the measured BPA concentrations and handling frequency of paper products. The daily intake of BPA (calculated from median concentrations) through dermal absorption from handling of papers was 17.5 and 1300 ng/day for the general population and occupationally exposed individuals, respectively; these values are minor compared with exposure through diet. Among paper products, thermal receipt papers contributed to the majority (>98%) of the exposures.
Abstract: Bisphenol A (BPA) is used in a variety of consumer products, including some paper products, particularly thermal receipt papers, for which it is used as a color developer. Nevertheless, little is known about the magnitude of BPA contamination or human exposure to BPA as a result of contact with paper and paper products. In this study, concentrations of BPA were determined in 15 types of paper products (n = 202), including thermal receipts, flyers, magazines, tickets, mailing envelopes, newspapers, food contact papers, food cartons, airplane boarding passes, luggage tags, printing papers, business cards, napkins, paper towels, and toilet paper, collected from several cities in the USA. Thermal receipt papers also were collected from Japan, Korea, and Vietnam. BPA was found in 94% of thermal receipt papers (n = 103) at concentrations ranging from below the limit of quantitation (LOQ, 1 ng/g) to 13.9 mg/g (geometric mean: 0.211 mg/g). The majority (81%) of other paper products (n = 99) contained BPA at concentrations ranging from below the LOQ to14.4 μg/g (geometric mean: 0.016 μg/g). Whereas thermal receipt papers contained the highest concentrations of BPA (milligram-per-gram), some paper products, including napkins and toilet paper, made from recycled papers contained microgram-per-gram concentrations of BPA. Contamination during the paper recycling process is a source of BPA in paper products. Daily intake (DI) of BPA through dermal absorption was estimated based on the measured BPA concentrations and handling frequency of paper products. The daily intake of BPA (calculated from median concentrations) through dermal absorption from handling of papers was 17.5 and 1300 ng/day for the general population and occupationally exposed individuals, respectively; these values are minor compared with exposure through diet. Among paper products, thermal receipt papers contributed to the majority (>98%) of the exposures.
http://dx.doi.org/10.1016/j.envres.2015.01.014/EnvironmentalResearch 142 (2015), 739-745 Bisphenol A exposure and behavioral problems among inner city children at 7–9 years of age
Abstract
Background: Bisphenol A (BPA) is a ubiquitous endocrine disrupting compound. Several experimental and epidemiological studies suggest that gestational BPA exposure can lead to neurodevelopmental and behavioral problems in early-life, but results have been inconsistent. We previously reported that prenatal BPA exposure may affect child behavior and differently among boys and girls at ages 3–5 years.
Objectives: We investigated the association of prenatal and early childhood BPA exposure with behavioral outcomes in 7–9 year old minority children and hypothesized that we would observe the same sex-specific pattern observed at earlier ages.
Methods: African-American and Dominican women enrolled in an inner-city prospective cohort study and their children were followed from mother’s pregnancy through children’s age 7–9 years. Women during the third trimester of pregnancy and children at ages 3 and 5 years provided spot urine samples. BPA exposure was categorized by tertiles of BPA urinary concentrations. The Child Behavioral Checklist (CBCL) was administered at ages 7 and 9 to assess multiple child behavior domains. Associations between behavior and prenatal (maternal) BPA concentrations and behavior and postnatal (child) BPA concentration were assessed via Poisson regression in models stratified by sex. These models accounted for potential confounders including prenatal or postnatal urinary BPA concentrations, child age at CBCL assessment, ethnicity, gestational age, maternal intelligence, maternal education and demoralization, quality of child’s home environment, prenatal environmental tobacco smoke exposure, and prenatal mono-n-butyl phthalate concentration.
http://dx.doi.org/10.1016/j.envint.2013.12.007, R. Rezg et al. / Environment International 64 (2014) 83–90
Bisphenol A and human chronic diseases: Current evidences, possible mechanisms, and future perspectives
Bisphenol-A (BPA) is one of the highest volume chemicals produced worldwide, with over 6 billion pounds produced and over 100 t released into the atmosphere each year. Recent extensive literature has raised concerns about its possible implication in the etiology of some human chronic diseases such as diabetes, obesity, reproductive disorders, cardiovascular diseases, birth defects, chronic respiratory and kidney diseases and breast cancer. In this review, we present the highlighted evidences on the relationship between BPA exposure and human chronic diseases and we discuss its eventual mechanisms of action, especially genetic, epigenetic and endocrine disruption mechanisms with the possible involvement of oxidative stress, mitochondrial dysfunction and cell signaling.
doi: 10.1016/j.jhazmat.2011.06.038, J Hazard Mater. 2011 Sep 15;192(3):1291-8.
Study on the interaction of phthalate esters to human serum albumin by steady-state and time-resolved fluorescence and circular dichroism spectroscopy.
Abstract: Phthalate esters (PAEs) are globally pervasive contaminants that are considered to be endocrine disruptor chemicals and toxic environmental priority pollutants. In this paper, the interactions between PAEs and human serum albumin (HSA) were examined by molecular modelling, steady state and time-resolved fluorescence, ultraviolet-visible spectroscopy (UV-vis) and circular dichroism spectroscopy (CD). The association constants between PAEs and HSA were determined using the Stern-Volmer and Scatchard equations. The binding of dimethyl phthalate (DMP) to HSA has a single class of binding site and its binding constants (K) are 4.08 × 103, 3.97 × 103, 3.45 × 103, and 3.20 × 103L mol-1 at 289, 296, 303, and 310K, respectively. The Stern-Volmer and Scatchard plots both had two regression curves for HSA-butylbenzyl phthalate (BBP) and HSA-di-2-ethylhexyl phthalate (DEHP), which indicated that these bindings were via two types of binding sites: the numbers of binding site for the first type were lower than for the second type. The binding constants of the first type binding site were higher than those of the second type binding site at corresponding temperatures, the results suggesting that the first type of binding site had high affinity and the second binding site involved other sites with lower binding affinity and selectivity. The thermodynamic parameters of the binding reactions (ΔG°, ΔH° and ΔS°) were measured, and they indicated the presences of hydrophobic forces and hydrogen interactions in the PAEs-HSA interactions, which agreed well with the results from molecular modelling. The alterations of protein secondary structure in the presence of PAEs were confirmed by UV-vis and CD spectroscopy. The time-resolved fluorescence study showed that the lifetime of Trp residue of HSA decreased after the addition of PAEs, which implied that the Trp residue of HSA was the main binding site.
doi:10.1016/j.jsbmb.2011.05.002/J Steroid Biochem Mol Biol. 2011 Oct;127(1-2):27-34.
Bisphenol A: an endocrine disruptor with widespread exposure and multiple effects.
Abstract
Bisphenol A (BPA) is one of the highest volume chemicals produced worldwide. This compound is a building block of polycarbonate plastics often used for food and beverage storage, and BPA is also a component of epoxy resins that are used to line food and beverage containers. Studies have shown that BPA can leach from these and other products in contact with food and drink, and as a result, routine ingestion of BPA is presumed. This compound is also found in an enormous number of other products that we come into contact with daily, and therefore it is not surprising that it has been detected in the majority of individuals examined. BPA is a known endocrine disruptor. Although initially considered to be a weak environmental estrogen, more recent studies have demonstrated that BPA may be similar in potency to estradiol in stimulating some cellular responses. Moreover, emerging evidence suggests that BPA may influence multiple endocrine-related pathways. Studies in rodents have identified adverse effects of BPA at levels at or below the current acceptable daily intake level for this compound. The various reported adverse effects of BPA are reviewed, and potential mechanisms of BPA action are discussed. Much more investigation is needed to understand the potential adverse health effects of BPA exposure in humans and to understand the multiple pathways through which it may act. Although many questions remain to be answered, it is becoming increasingly apparent that exposure to BPA is ubiquitous and that the effects of this endocrine disruptor are complex and wide-ranging.
doi.org/10.1021/es300876n | Environ. Sci. Technol. 2012, 46, 6515−6522 Bisphenol S, a New Bisphenol Analogue, in Paper Products and Currency Bills and Its Association with Bisphenol A Residues
As the evidence of the toxic effects of bisphenol A (BPA) grows, its application in commercial products is gradually being replaced with other related compounds, such as bisphenol S (BPS). Nevertheless, very little is known about the occurrence of BPS in the environment. In this study, BPS was analyzed in 16 types of paper and paper products (n = 268), including thermal receipts, paper currencies, flyers, magazines, newspapers, food contact papers, airplane luggage tags, printing paper, kitchen rolls (i.e., paper towels), and toilet paper. All thermal receipt paper samples (n = 111) contained BPS at concentrations ranging from 0.0000138 to 22.0 mg/g (geometric mean: 0.181 mg/g). The overall mean concentrations of BPS in thermal receipt papers were similar to the concentrations reported earlier for BPA in the same set of samples. A significant negative correlation existed between BPS and BPA concentrations in thermal receipt paper samples (r = −0.55, p < 0.0001). BPS was detected in 87% of currency bill samples (n = 52) from 21 countries, at concentrations ranging from below the limit of quantification (LOQ) to 6.26 μg/g (geometric mean: 0.029 μg/g). BPS also was found in 14 other paper product types (n = 105), at concentrations ranging from < LOQ to 8.38 μg/g (geometric mean: 0.0036 μg/g; detection rate: 52%). The estimated daily intake (EDI) of BPS, through dermal absorption via handling of papers and currency bills, was estimated on the basis of concentrations and frequencies of the handling of papers by humans. The median and 95th percentile EDI values, respectively, were 4.18 and 11.0 ng/kg body weight (bw)/day for the general population and 312 and 821 ng/kg bw/day for occupationally exposed individuals. Among the paper types analyzed, thermal receipt papers were found to be the major sources of human exposure to BPS (>88%). To our knowledge, this is the first report on the occurrence of BPS in paper products and currency bills.
doi:10.1016/j.chemosphere.2011.06.060, J.E. Cooper et al. / Chemosphere 85 (2011) 943–947 Assessment of bisphenol A released from reusable plastic, aluminium and stainless steel water bottles
Abstract: Bisphenol A (BPA) is a ubiquitous high volume industrial chemical that is an estrogen and an environmental endocrine disrupting chemical. Bisphenol A is used extensively in the production of consumer goods, polycarbonate plastics, epoxy resins and coatings used to line metallic food and beverage cans. There is great concern regarding the possible harmful effects from exposures that result from BPA leaching into foods and beverages from packaging or storage containers. The objective of this study was to independently assess whether BPA contamination of water was occurring from different types of reusable drinking bottles marketed as alternatives to BPA-containing polycarbonate plastics. Using a sensitive and quantitative BPA-specific competitive enzyme-linked immunosorbent assay we evaluated whether BPA migrated into water stored in polycarbonate or copolyester plastic bottles, and different lined or unlined metallic reusable water bottles. At room temperature the concentration of BPA migrating from polycarbonate bottles ranged from 0.2 to 0.3 mg L-1. Under identical conditions BPA migration from aluminium bottles lined with epoxy-based resins was variable depending on manufacturer ranging from 0.08 to 1.9 mg L-1. Boiling water significantly increased migration of BPA from the epoxy lined bottles. No detectable BPA contamination was observed in water stored in bottles made from Tritan™ copolyester plastic, uncoated stainless steel, or aluminium lined with EcoCare™. The results from this study demonstrate that when used according to manufacturers’ recommendations reusable water bottles constructed from ‘‘BPA-free’’ alternative materials are suitable for consumption of beverages free of BPA contamination.
dx.doi.org/10.1021/es202507f | Environ. Sci. Technol. 2011, 45, 9372–9379 Widespread Occurrence of Bisphenol A in Paper and Paper Products: Implications for Human Exposure

http://dx.doi.org/10.1016/j.envres.2015.01.014/EnvironmentalResearch 142 (2015), 739-745 Bisphenol A exposure and behavioral problems among inner city children at 7–9 years of age
Abstract
Background: Bisphenol A (BPA) is a ubiquitous endocrine disrupting compound. Several experimental and epidemiological studies suggest that gestational BPA exposure can lead to neurodevelopmental and behavioral problems in early-life, but results have been inconsistent. We previously reported that prenatal BPA exposure may affect child behavior and differently among boys and girls at ages 3–5 years.
Objectives: We investigated the association of prenatal and early childhood BPA exposure with behavioral outcomes in 7–9 year old minority children and hypothesized that we would observe the same sex-specific pattern observed at earlier ages.
Methods: African-American and Dominican women enrolled in an inner-city prospective cohort study and their children were followed from mother’s pregnancy through children’s age 7–9 years. Women during the third trimester of pregnancy and children at ages 3 and 5 years provided spot urine samples. BPA exposure was categorized by tertiles of BPA urinary concentrations. The Child Behavioral Checklist (CBCL) was administered at ages 7 and 9 to assess multiple child behavior domains. Associations between behavior and prenatal (maternal) BPA concentrations and behavior and postnatal (child) BPA concentration were assessed via Poisson regression in models stratified by sex. These models accounted for potential confounders including prenatal or postnatal urinary BPA concentrations, child age at CBCL assessment, ethnicity, gestational age, maternal intelligence, maternal education and demoralization, quality of child’s home environment, prenatal environmental tobacco smoke exposure, and prenatal mono-n-butyl phthalate concentration.
http://dx.doi.org/10.1016/j.envint.2013.12.007, R. Rezg et al. / Environment International 64 (2014) 83–90
Bisphenol A and human chronic diseases: Current evidences, possible mechanisms, and future perspectives
Bisphenol-A (BPA) is one of the highest volume chemicals produced worldwide, with over 6 billion pounds produced and over 100 t released into the atmosphere each year. Recent extensive literature has raised concerns about its possible implication in the etiology of some human chronic diseases such as diabetes, obesity, reproductive disorders, cardiovascular diseases, birth defects, chronic respiratory and kidney diseases and breast cancer. In this review, we present the highlighted evidences on the relationship between BPA exposure and human chronic diseases and we discuss its eventual mechanisms of action, especially genetic, epigenetic and endocrine disruption mechanisms with the possible involvement of oxidative stress, mitochondrial dysfunction and cell signaling.
doi: 10.1016/j.jhazmat.2011.06.038, J Hazard Mater. 2011 Sep 15;192(3):1291-8.
Study on the interaction of phthalate esters to human serum albumin by steady-state and time-resolved fluorescence and circular dichroism spectroscopy.
Abstract: Phthalate esters (PAEs) are globally pervasive contaminants that are considered to be endocrine disruptor chemicals and toxic environmental priority pollutants. In this paper, the interactions between PAEs and human serum albumin (HSA) were examined by molecular modelling, steady state and time-resolved fluorescence, ultraviolet-visible spectroscopy (UV-vis) and circular dichroism spectroscopy (CD). The association constants between PAEs and HSA were determined using the Stern-Volmer and Scatchard equations. The binding of dimethyl phthalate (DMP) to HSA has a single class of binding site and its binding constants (K) are 4.08 × 103, 3.97 × 103, 3.45 × 103, and 3.20 × 103L mol-1 at 289, 296, 303, and 310K, respectively. The Stern-Volmer and Scatchard plots both had two regression curves for HSA-butylbenzyl phthalate (BBP) and HSA-di-2-ethylhexyl phthalate (DEHP), which indicated that these bindings were via two types of binding sites: the numbers of binding site for the first type were lower than for the second type. The binding constants of the first type binding site were higher than those of the second type binding site at corresponding temperatures, the results suggesting that the first type of binding site had high affinity and the second binding site involved other sites with lower binding affinity and selectivity. The thermodynamic parameters of the binding reactions (ΔG°, ΔH° and ΔS°) were measured, and they indicated the presences of hydrophobic forces and hydrogen interactions in the PAEs-HSA interactions, which agreed well with the results from molecular modelling. The alterations of protein secondary structure in the presence of PAEs were confirmed by UV-vis and CD spectroscopy. The time-resolved fluorescence study showed that the lifetime of Trp residue of HSA decreased after the addition of PAEs, which implied that the Trp residue of HSA was the main binding site.
doi:10.1016/j.jsbmb.2011.05.002/J Steroid Biochem Mol Biol. 2011 Oct;127(1-2):27-34.
Bisphenol A: an endocrine disruptor with widespread exposure and multiple effects.
Abstract
Bisphenol A (BPA) is one of the highest volume chemicals produced worldwide. This compound is a building block of polycarbonate plastics often used for food and beverage storage, and BPA is also a component of epoxy resins that are used to line food and beverage containers. Studies have shown that BPA can leach from these and other products in contact with food and drink, and as a result, routine ingestion of BPA is presumed. This compound is also found in an enormous number of other products that we come into contact with daily, and therefore it is not surprising that it has been detected in the majority of individuals examined. BPA is a known endocrine disruptor. Although initially considered to be a weak environmental estrogen, more recent studies have demonstrated that BPA may be similar in potency to estradiol in stimulating some cellular responses. Moreover, emerging evidence suggests that BPA may influence multiple endocrine-related pathways. Studies in rodents have identified adverse effects of BPA at levels at or below the current acceptable daily intake level for this compound. The various reported adverse effects of BPA are reviewed, and potential mechanisms of BPA action are discussed. Much more investigation is needed to understand the potential adverse health effects of BPA exposure in humans and to understand the multiple pathways through which it may act. Although many questions remain to be answered, it is becoming increasingly apparent that exposure to BPA is ubiquitous and that the effects of this endocrine disruptor are complex and wide-ranging.
doi.org/10.1021/es300876n | Environ. Sci. Technol. 2012, 46, 6515−6522 Bisphenol S, a New Bisphenol Analogue, in Paper Products and Currency Bills and Its Association with Bisphenol A Residues
As the evidence of the toxic effects of bisphenol A (BPA) grows, its application in commercial products is gradually being replaced with other related compounds, such as bisphenol S (BPS). Nevertheless, very little is known about the occurrence of BPS in the environment. In this study, BPS was analyzed in 16 types of paper and paper products (n = 268), including thermal receipts, paper currencies, flyers, magazines, newspapers, food contact papers, airplane luggage tags, printing paper, kitchen rolls (i.e., paper towels), and toilet paper. All thermal receipt paper samples (n = 111) contained BPS at concentrations ranging from 0.0000138 to 22.0 mg/g (geometric mean: 0.181 mg/g). The overall mean concentrations of BPS in thermal receipt papers were similar to the concentrations reported earlier for BPA in the same set of samples. A significant negative correlation existed between BPS and BPA concentrations in thermal receipt paper samples (r = −0.55, p < 0.0001). BPS was detected in 87% of currency bill samples (n = 52) from 21 countries, at concentrations ranging from below the limit of quantification (LOQ) to 6.26 μg/g (geometric mean: 0.029 μg/g). BPS also was found in 14 other paper product types (n = 105), at concentrations ranging from < LOQ to 8.38 μg/g (geometric mean: 0.0036 μg/g; detection rate: 52%). The estimated daily intake (EDI) of BPS, through dermal absorption via handling of papers and currency bills, was estimated on the basis of concentrations and frequencies of the handling of papers by humans. The median and 95th percentile EDI values, respectively, were 4.18 and 11.0 ng/kg body weight (bw)/day for the general population and 312 and 821 ng/kg bw/day for occupationally exposed individuals. Among the paper types analyzed, thermal receipt papers were found to be the major sources of human exposure to BPS (>88%). To our knowledge, this is the first report on the occurrence of BPS in paper products and currency bills.
